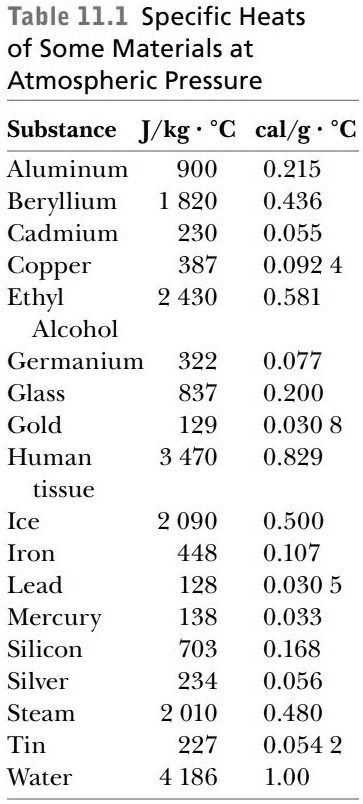
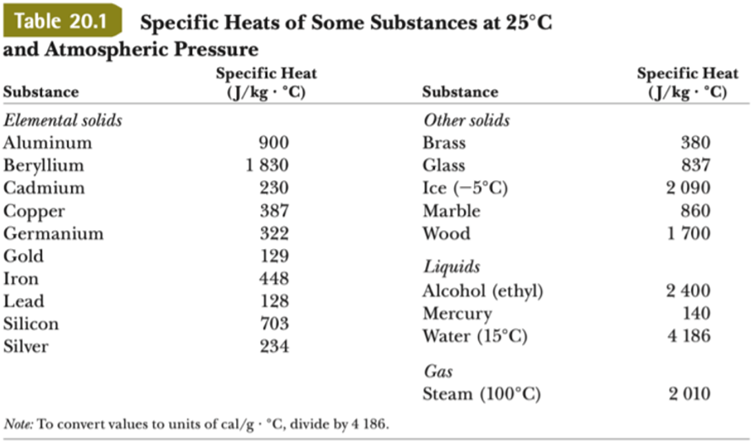
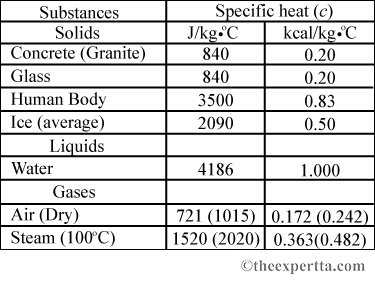
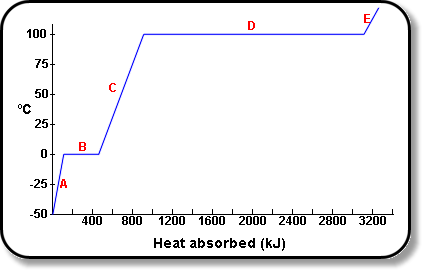
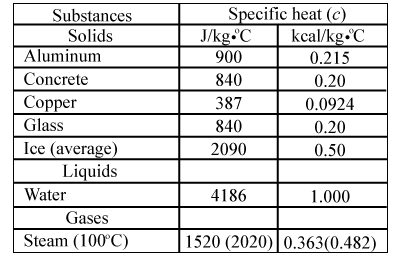
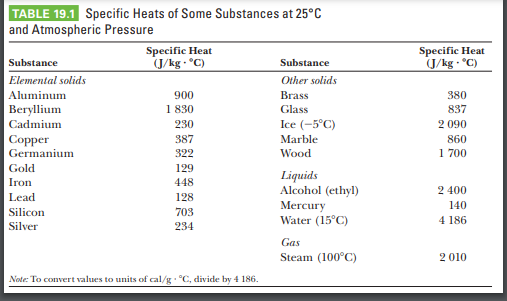
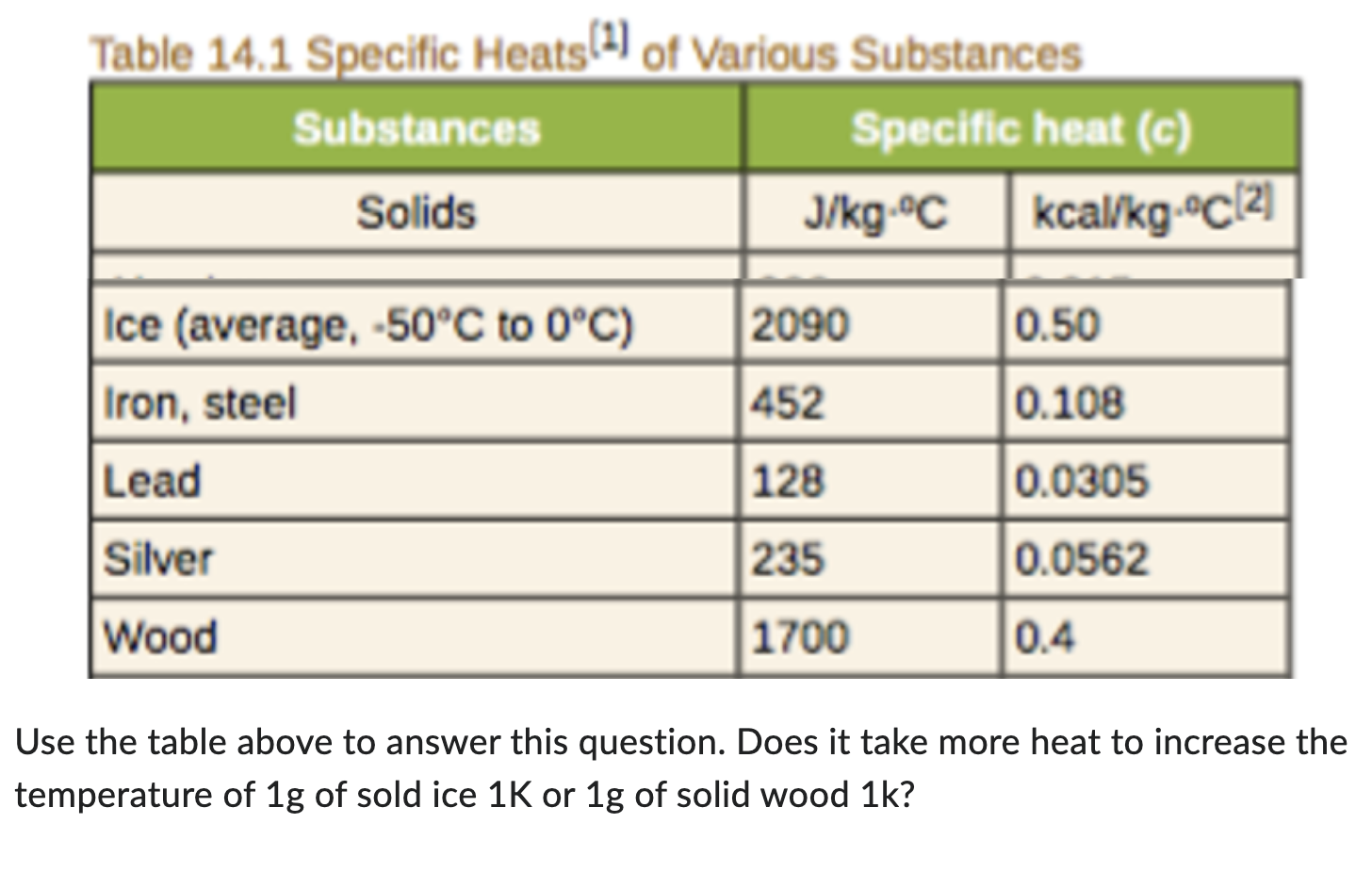
Heat energy of 184 kJ is given to ice of mass 600 g at -12°C, Specific heat of ice is 2222.3 J - Sarthaks eConnect | Largest Online Education Community

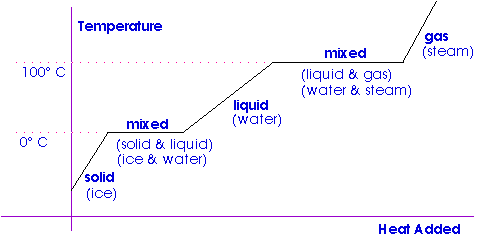
1 PHYS1001 Physics 1 REGULAR Module 2 Thermal Physics HEAT CAPACITY LATENT HEAT What is cooking all about? ptC_heat.ppt. - ppt download
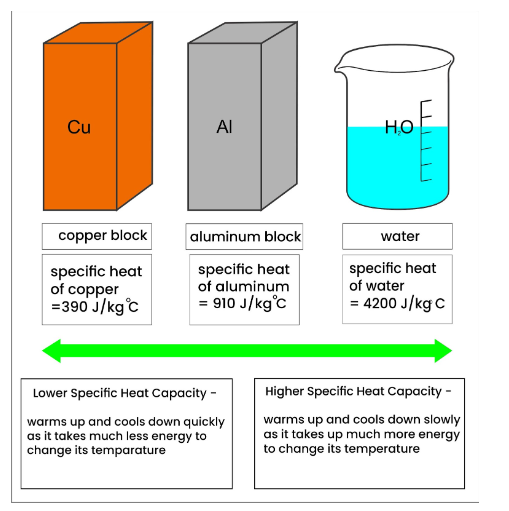
What is the difference between specific latent heat of melting of ice and specific latent heat of fusion of ice? - Quora

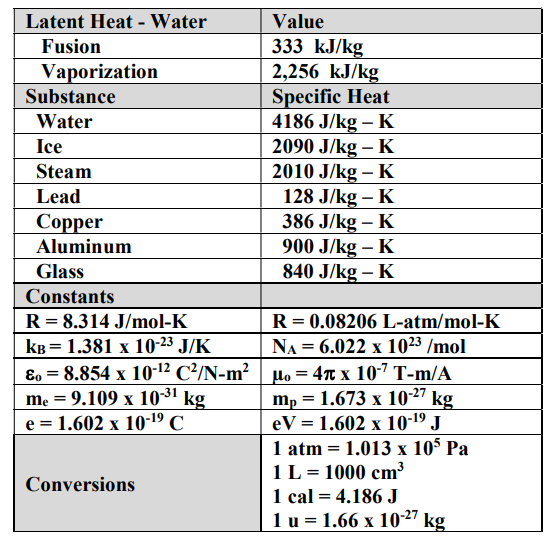
A 40 g ice cube (latent heat of fusion = 3.33 x 10^5 J/kg) floats in 200 g of water (specific heat = 4184 J/kg degree Celsius) in a 100 g copper (