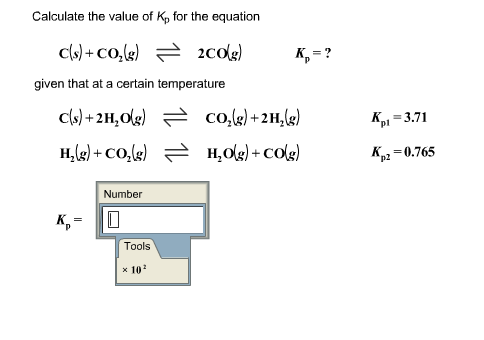
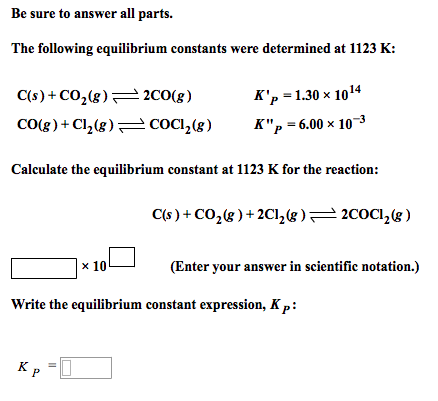
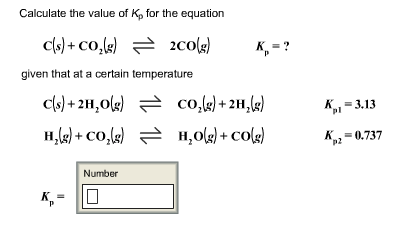
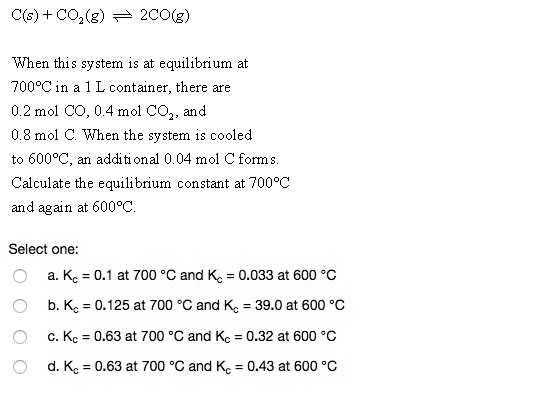
Solve this: â ‹Q3 For the equilibrium C(s) + CO2(g) ⇌2CO(g) KP = 63 atm at 1000 K - Chemistry - Chemistry in Everyday Life - 11997217 | Meritnation.com

Equilibrium concentrations of reactants and products in the reaction C... | Download Scientific Diagram
The value of K for the reaction Co2(g) + C(s) (eqi. symbol) 2Co is 3.0 at 1000K If initially pCO=0.48 bar and pCO= 0 bar and pure graphite is present. calculate the
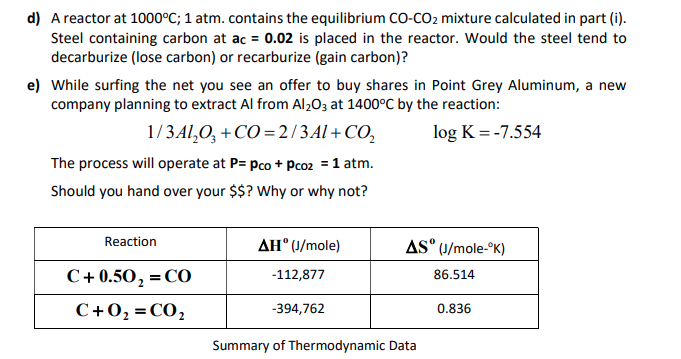
3.For the equilibrium C(s)+ COg) gives 2CO(g) Kp is 63 atm at 1000 K. If at equilibrium Pco=10Pco2 then total pressure at equilibrium is : 4.A(g) is 90

For the reaction, c(s)+co2(g)⇌2co(g), the partial pressures of co2 and co are 2.0 and 4.0 atm respectively - Brainly.in

Given, 2CO(g) C(s) + CO2(g); Kp1 = 10^-14atm^-1 at 1120 K CO(g) + Cl2(g) COCl2(g); Kp2 = 6 × 10^-3 atm^-1 The value of Kc for the following reaction at 1120 K

In the reaction C(s) + CO2(g) 2CO(g) the equilibrium pressure is 12 atm. If 50% of CO2 reacts calculate Kp .
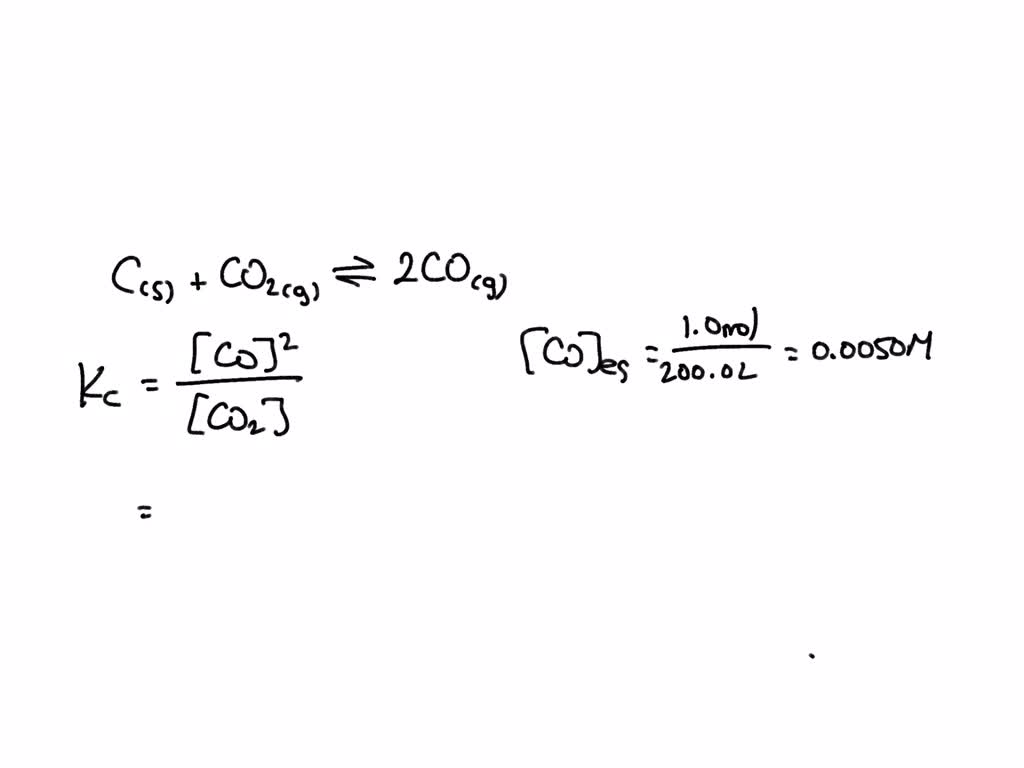
SOLVED: The reaction C(s) + CO2(g) ⇔ 2CO(g) occurs at high temperatures. At 700°C, a 200.0 L tank contains 1.0 mol CO, 0.20 mol CO2, and 0.40 mol of C at equilibrium.

For the reaction, C (s) + CO2 (g) 2CO (g) , the partial pressures of CO2 and CO are 2.0 and 4.0 atm respectively at equilibrium. The Kp for the reaction is: